Ceiling Price Lookup
-
To look up ceiling prices, click Pricing in the “What Would You Like to Do?” section or click the Pricing tab on the menu bar of the
 340B OPAIS The 340B Office of Pharmacy Affairs Information System (OPAIS) is a collection of information submitted by covered entities, contract pharmacies, and manufacturers maintained and verified by HRSA's Office of Pharmacy Affairs (OPA). home page
340B OPAIS The 340B Office of Pharmacy Affairs Information System (OPAIS) is a collection of information submitted by covered entities, contract pharmacies, and manufacturers maintained and verified by HRSA's Office of Pharmacy Affairs (OPA). home page -
Upon selecting the Pricing function, the system will prompt you to log in and authenticate again to gain access to the Pricing data.
-
Upon a successful login, the system displays the
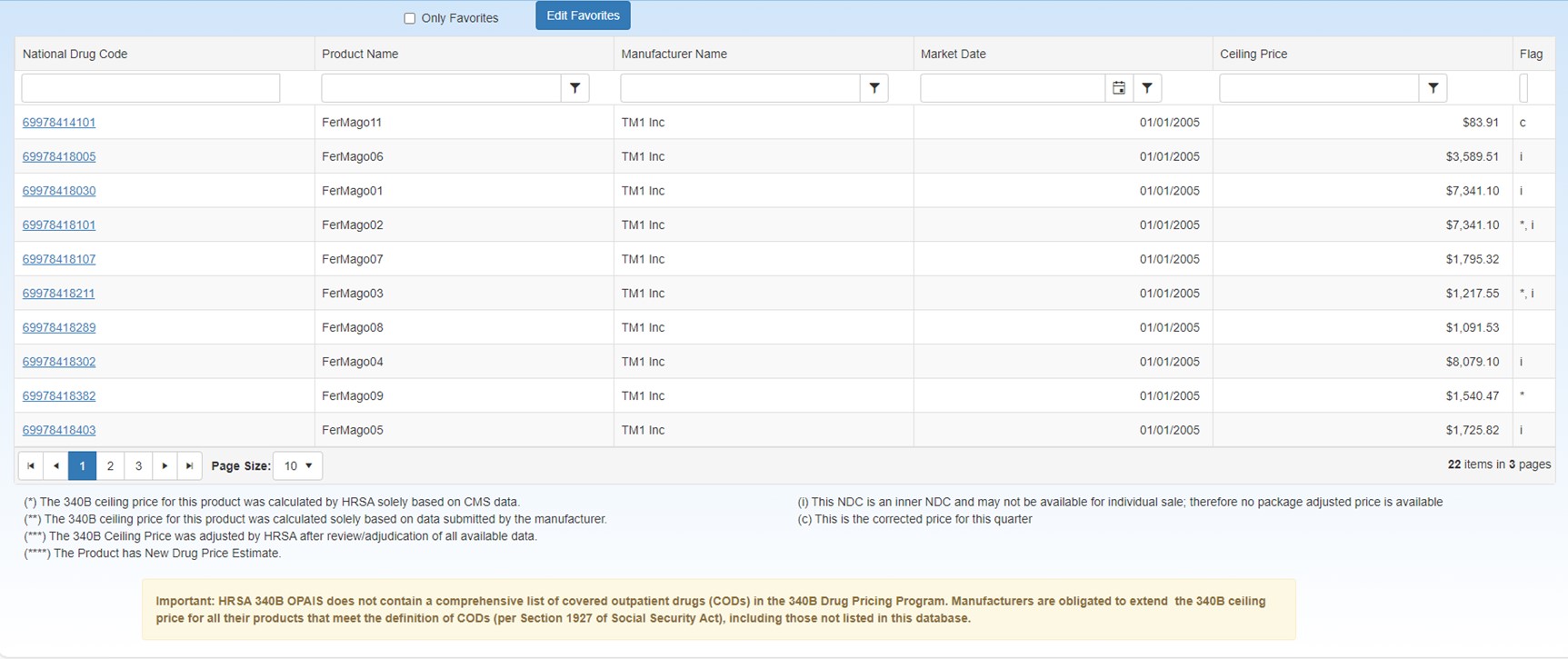
 Ceiling Price Manufacturers who participate in the Medicaid Drug Rebate Program (MDRP) enter into an agreement with the Secretary of Health and Human Services under which the manufacturer must agree to charge a price no greater than the statutory pricing formula (340B ceiling price) when selling covered outpatient drugs to 340B covered entities. In order to calculate the 340B ceiling price, the Unit Rebate Amount (URA) is subtracted from the Average Manufacturer Price (AMP) for the smallest unit of measure [340B Ceiling Price = (AMP – URA)]. Under section 340B(a) of the Public Health Service Act (PHSA), the 340B ceiling price is calculated by subtracting the unit rebate amount (URA) from the average manufacturer price (AMP) for the smallest unit of measure of each covered outpatient drug (as identified by the product's 11-digit National Drug Code (NDC). To ensure that the final price is operational in the marketplace, HRSA then multiplies this amount by drug's package size (PS), defined as the number of billing units in the labeled quantity from which the pharmacist dispenses, and the case pack size (CSP), defined as the number of salable units in the shipping container [340B Ceiling Price = (AMP-URA) x PS x CSP]. page. This table can be sorted and filtered to make the data displayed more manageable. For more information, refer to Data Tables.
Ceiling Price Manufacturers who participate in the Medicaid Drug Rebate Program (MDRP) enter into an agreement with the Secretary of Health and Human Services under which the manufacturer must agree to charge a price no greater than the statutory pricing formula (340B ceiling price) when selling covered outpatient drugs to 340B covered entities. In order to calculate the 340B ceiling price, the Unit Rebate Amount (URA) is subtracted from the Average Manufacturer Price (AMP) for the smallest unit of measure [340B Ceiling Price = (AMP – URA)]. Under section 340B(a) of the Public Health Service Act (PHSA), the 340B ceiling price is calculated by subtracting the unit rebate amount (URA) from the average manufacturer price (AMP) for the smallest unit of measure of each covered outpatient drug (as identified by the product's 11-digit National Drug Code (NDC). To ensure that the final price is operational in the marketplace, HRSA then multiplies this amount by drug's package size (PS), defined as the number of billing units in the labeled quantity from which the pharmacist dispenses, and the case pack size (CSP), defined as the number of salable units in the shipping container [340B Ceiling Price = (AMP-URA) x PS x CSP]. page. This table can be sorted and filtered to make the data displayed more manageable. For more information, refer to Data Tables.
| Control | Description |
|---|---|
| Only Favorites |
|
| Edit Favorites | Clicking this button displays a pop-up window to let you enter a list of favorite NDCs. |
| National Drug Code | Select a link in the National Drug Codes column to
view the Product Detail page for that |
| Ceiling Price | Calculated by subtracting the unit rebate amount
( |
| Package Adjusted Price | Calculated by multiplying the Ceiling Price amount by
the drug’s Package Size ( |
| Flag |
The asterisks in the Flag column indicate that HRSA had only one source of information for determining the 340B ceiling price. The full meaning for the asterisks is shown at the bottom of the page: (*) The 340B ceiling price for this product was calculated by HRSA solely based on (**) The 340B ceiling price for this product was calculated by HRSA solely based on using only data submitted by the manufacturer. No CMS pricing data was available for ceiling price comparison. HRSA publishes the 340B ceiling price without comparing it with the AMP and URA data points received from CMS in situations where the manufacturer does not participate in the Medicaid Drug Rebate Program and CMS has no quarterly pricing data to report for the specified product (***) The 340B ceiling price was adjusted by HRSA after review/adjudication of all available data. HRSA publishes the 340B ceiling price after the data points were adjusted by HRSA during review/adjudication. (*****) The product is a New Drug Price Estimate. HRSA publishes the 340B ceiling price for the product that has been marked as a New Drug Price Estimate. (c) – This is the corrected price for this quarter |